There are two techniques in measuring radiocarbon in samples—through radiometric dating and by Accelerator Mass Spectrometry (AMS). The two techniques are used primarily in determining carbon 14 content of archaeological artifacts and geological samples. These two radiocarbon dating methods use modern standards such as oxalic acid and other reference materials. Although both radiocarbon dating methods produce high-quality results, they are fundamentally different in principle.
Radiometric dating methods detect beta particles from the decay of carbon 14 atoms while accelerator mass spectrometers count the number of carbon 14 atoms present in the sample. Both carbon dating methods have advantages and disadvantages.
Mass spectrometers detect atoms of specific elements according to their atomic weights. They, however, do not have the sensitivity to distinguish atomic isobars (atoms of different elements that have the same atomic weight, such as in the case of carbon 14 and nitrogen 14—the most common isotope of nitrogen).
Thanks to nuclear physics, mass spectrometers have been fine-tuned to separate a rare isotope from an abundant neighboring mass, and accelerator mass spectrometry was born. A method has finally been developed to detect carbon 14 in a given sample and ignore the more abundant isotopes that swamp the carbon 14 signal.
There are essentially two parts in the process of radiocarbon dating through accelerator mass spectrometry. The first part involves accelerating the ions to extraordinarily high kinetic energies, and the subsequent step involves mass analysis.
There are two accelerator systems commonly used for radiocarbon dating through accelerator mass spectrometry. One is the cyclotron, and the other is a tandem electrostatic accelerator.
Disclaimer: This video is hosted in a third-party site and may contain advertising.
After pretreatment, samples for radiocarbon dating are prepared for use in an accelerator mass spectrometer by converting them into a solid graphite form. This is done by conversion to carbon dioxide with subsequent graphitization in the presence of a metal catalyst. Burning the samples to convert them into graphite, however, also introduces other elements into the sample like nitrogen 14.
When the samples have finally been converted into few milligrams of graphite, they are pressed on to a metal disc. Reference materials are also pressed on metal discs. These metal discs are then mounted on a target wheel so they can be analyzed in sequence.
Ions from a cesium gun are then fired at the target wheel, producing negatively ionized carbon atoms. These negatively ionized carbon atoms pass through focusing devices and an injection magnet before reaching the tandem accelerator where they are accelerated to the positive terminal by a voltage difference of two million volts.
At this stage, other negatively charged atoms are unstable and cannot reach the detector. The negatively charged carbon atoms, however, move on to the stripper (a gas or a metal foil) where they lose the electrons and emerge as the triple, positively charged carbon atoms. At this stage, molecules that may be present are eliminated because they cannot exist in this triple charged state.
The carbon atoms with triple positive charge further accelerate away from the positive terminal and pass through another set of focusing devices where mass analysis occurs.
In mass analysis, a magnetic field is applied to these moving charged particles, which causes the particles to deflect from the path they are traveling. If the charged particles have the same velocity but different masses, as in the case of the carbon isotopes, the heavier particles are deflected least. Detectors at different angles of deflection then count the particles.
At the end of an AMS run, data gathered is not only the number of carbon 14 atoms in the sample but also the quantity of carbon 12 and carbon 13. From these data, concentration ratio of the isotopes can be known to allow evaluation of the level of fractionation.
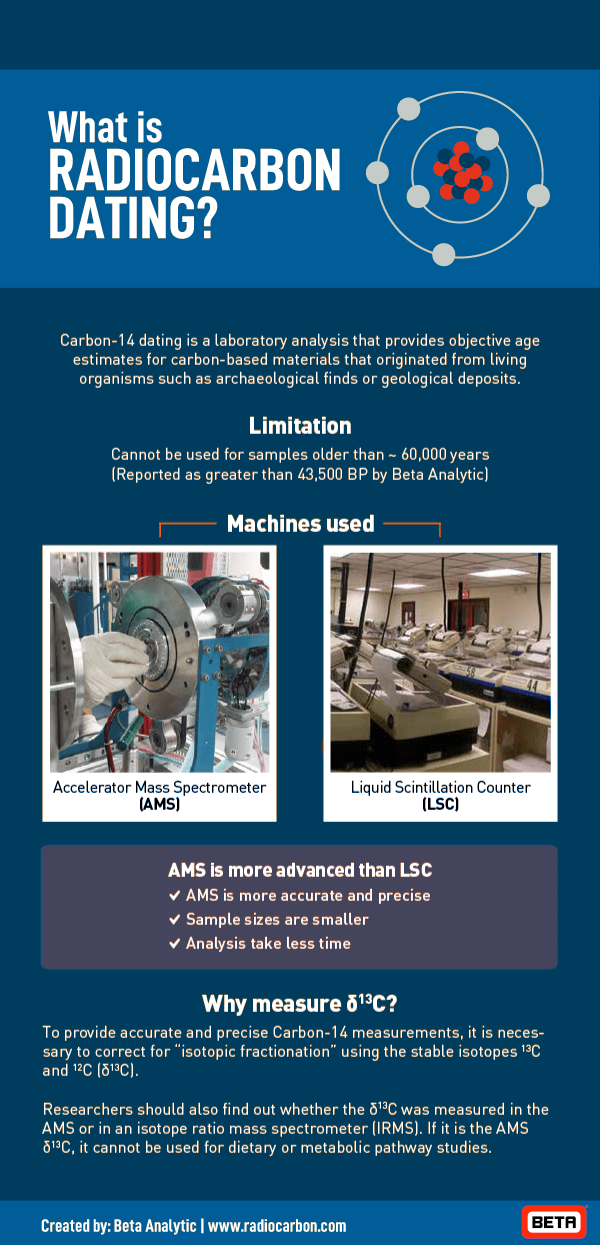
The greatest advantage that AMS radiocarbon dating has over radiometric methods is small sample size. Accelerator mass spectrometers need only as little as 20 milligrams and as high as 500 milligrams for certain samples whereas conventional methods need at least 10 grams in samples like wood and charcoal and as much as 100 grams in bones and sediments. Accelerator mass spectrometers typically need sample sizes lesser than conventional methods by a factor of 1,000.
Radiocarbon dating is a destructive process. Hence, because of its ability to analyze samples even in minute amounts, accelerator mass spectrometry is the method of choice for archaeologists with small artifacts and those who cannot destroy very expensive or rare materials.
Due to the sensitivity of accelerator mass spectrometers, carbon dating small particles like blood particles, a grain, or a seed have been made possible.
Accelerator mass spectrometry also takes less time to analyze samples for carbon 14 content compared to radiometric dating methods that can take one or two days. An accelerator mass spectrometer has a run time of a few hours per sample.
Lastly, it must be noted that AMS measurements usually achieve higher precision and lower backgrounds than radiometric dating methods.
An accelerator mass spectrometer, although a powerful tool, is also a costly one. Establishing and maintaining an accelerator mass spectrometer costs millions of dollars.
Due to the small sample sizes involved, control of contaminants is also difficult. Rigorous pretreatment is needed to make sure contaminants have been eliminated and will not lead to substantial errors during the carbon dating process.
Aside from archaeology, geology and ocean sciences research, AMS is used by biomedical laboratories using “hot” samples labeled with 14C for drug discovery.
Accelerator mass spectrometers are also used in pharmacokinetics, metabolite profiling, toxicology, and microdosing.
AMS is used to determine the natural abundance levels of carbon 14 in oceans as well as to carbon date sedimentary deposits. Accelerator mass spectrometry was used in building a three-dimensional map of carbon 14 distribution in dissolved inorganic carbon.
Beta Analytic White Paper on AMS Dating (PDF)
Disclaimer: This video is hosted in a third-party site and may contain advertising.
This video excerpt is part of Beta Analytic’s webinar: Isotopes 101: An Introduction to Isotopic Analysis